15 requirements you need to find in a solid eQMS for GDP Logisitics
- Step-by-step guidance and checklist on 15 non-negotiable GDP requirements
- How to avoid common pitfalls in pharmaceutical distribution
- Expert insights from industry leaders to become and stay GDP audit-ready
- ...
Download the Ebook

Why this Ebook is essential for your team
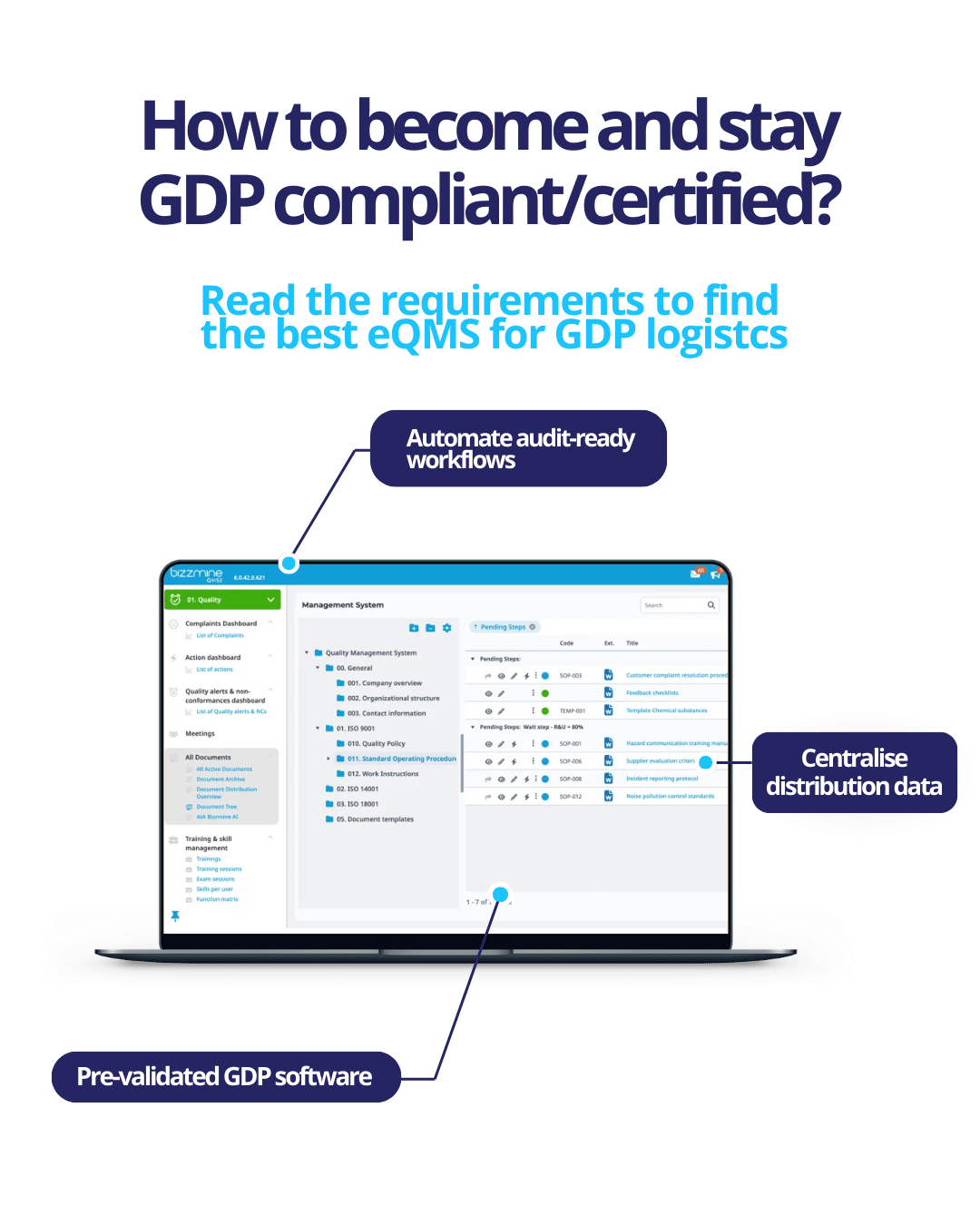
Ensure audit-ready compliance
Discover how a digital QMS automates GDP workflows from temperature monitoring to electronic batch records, to eliminate manual errors, reduce inspection risks, and maintain compliance with evolving regulations.
Centralize critical distribution data
Learn how integrating logistics, quality controls, and risk assessments into a single platform centralises data, enhances visibility, and empowers data-driven decisions to safeguard product integrity.
Strengthen collaboration
See how a unified system streamlines communication between suppliers, distributors, and quality teams, ensuring seamless documentation and alignment with ISO/GDP standards.
The industries' best action points on how to improve your GDP Logistics compliance.