Digital QMS
BizzMine Quality Management is easy to set up, without technical knowledge.
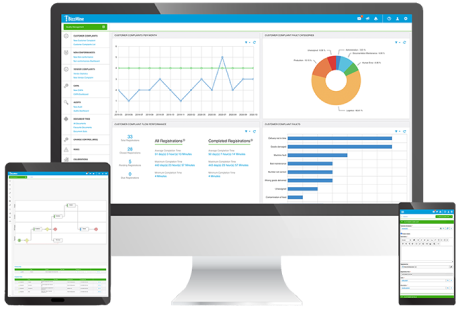
The software works on all devices and is available in the Cloud or On-Premises.
Pre-validated platform
All users have easy access to the software, no matter where they work and you have full control and overview over your entire Quality Management.
With BizzMine, your quality processes fully comply with ISO 9001, ISO 13485, ISO 17025, ISO 15189, ISO/TS 16949, ISO 22000, 21CFR Part 11, GxP, and GAMP.
It has been proved that BizzMine is able to be qualified for use in a pharmaceutical environment.

Get started fast
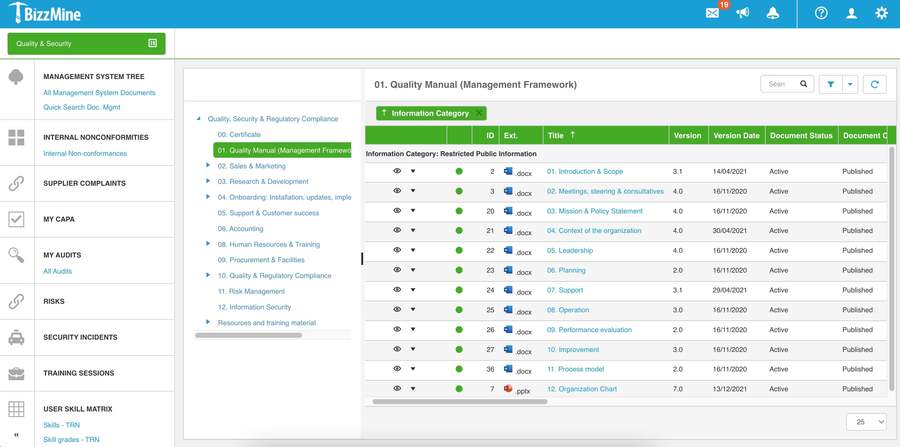
With the prebuilt processes you can implement your quality management in a couple of days.
Configure existing or new processes, without any need for coding.
Make changes yourself or count on our business consultants for help.
Go for the cloud or On-Premises. Choose for maximum comfort and security.

Scalable and flexible
Scale from a small business to a large one, spread over multiple sites. BizzMine grows easily with your business.
Expand your digital workflow approach to other departments of your organization.
Use BizzMine in English, French, Dutch, German, Swedish, Portuguese, Lithuanian, or Italian. More languages can be offered upon request.
Trust our support around the globe, since we have offices on all continents.

A centralised platform helps you ensure compliance with specific regulations and guidelines.
Increased efficiency
Automate and link all quality processes: document control and management of change, CAPA, risks, complaints, audits...
Facilitate collaboration among stakeholders, such as quality assurance, manufacturing, and regulatory affairs teams.
Analytics and reporting help you identify trends in quality data and continuously improve processes.
Real-time visibility lets you quickly identify issues and prevent negative impacts on product safety and efficacy.
Register your data on any device: laptop, tablet or smartphone. Go for cloud or on-premises.


























