Digital QMS for Laboratories
Improve the quality and safety of your laboratory and get fast and reliable results through the implementation of a Digital Quality Management System.
Optimise Laboratory operations with our software for QMS

Streamline operations
Leverage our QMS software to enhance laboratory efficiency. Manage quality controls and reduce errors with automated workflows, ensuring compliance with global standards. Achieve faster turnaround times, increased reliability, and greater accuracy in your laboratory processes.

Full Compliance
Stay audit-ready with easily traceable and auditable laboratory quality processes. With compliance to ISO 17025, ISO 15189, and ISO 14001 Bizzmine ensures data integrity, security, and full regulatory adherence. Minimise non-compliance risks and ensure seamless certifications and inspections.

Improve Data Integrity
Build a better risk management system designed to identify, assess, monitor, and treat risks effectively. Tailored to your specific needs for seamless quality management, our software enhances your laboratory performance in the shortest time
"The fact that we can configure any process in Bizzmine,
makes it very easy to automate a lot of our processes and document them."
- Anwa Medical Labs
Our Laboratory Quality Management System
Innovation and high-quality solutions are the keys to the most accurate laboratory results in the shortest time. Highly trained laboratory personnel take care of various analyses used in a medical and pharmaceutical environment, or in the food and agricultural industry.
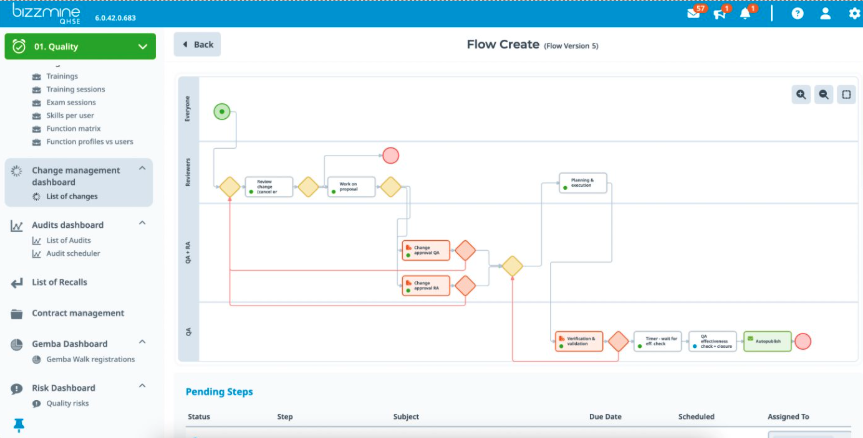
Implementing a decent quality management system helps you to digitise and organise numerous activities: Internal and external Audit Management, Risk Management, Corrective and Preventive Action (CAPA), Incident Management, Complaint Management, Task Management, Document Control, and Calibration Management.
Compliance ensured
Any organisation that performs testing, sampling, or calibration wants reliable results and strives for a high level of standardisation of testing required by auditors.
With Bizzmine you have a validated QMS software that complies with international standards.
Measure up to stringent legal and regulatory requirements and meet all quality requirements and regulations according to ISO standards for ISO /IEC 17025, ISO 15189, and ISO 14001 in a flexible platform.
Streamline your operations, become more resilient and build a sustainable business. This is how Bizzmine can be your powerful business improvement tool.

eQMS for Forensics/Labs
We have extensive experience with working with Forensics Labs within constabularies and private labs.
Bizzmine is the perfect solution for managing time consuming QMS processes to comply with ISO 17025, ISO 17020, ISO 9001, FSR.
These are just some of the modules we cover: Document Control and Document Changes, CAPA, Audits, Risk Management, Complaints, Staff Feedback and Positive Outcomes, Change Control, Meeting Management, Training and Exams.

Laboratory compliant software
With our solution, you can measure up to stringent legal and regulatory requirements and meet all quality requirements and regulations according to ISO standards for ISO /IEC 17025, ISO 15189, and ISO 14001 in a flexible platform.
Pre-validated software solution, tailor made for laboratories.
How can we help with your compliance?
What to expect:
Introduction about the platform and its functions to comply with regulations
Advice and onboarding with our most-fitting template/demo for your business context
Personal answers to your detailed questions about compliance software for your organisation
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)







/Customer_Health%20in%20Code_Logo.jpg)



/Ebook%20cover_Digital%20QMS_EN.png)
