Audit management software
Stay compliant with your industry-specific standards, leverage audit software to enhance oversight, and identify opportunities for improvement within your QMS.
Be prepared for audits with audit software
By centralising all records, you can ensure full traceability and be prepared for successful internal and external audits with audit management tools.
Register all relevant data in the customisable audit form and attach (scanned) reports or images to document the findings.
You can use practical checklists during audits to verify compliance with safety and quality guidelines. They help ensure that every audit is consistent, accurate and complete and can be accessed from any mobile device, making them ideal for audits in the field.
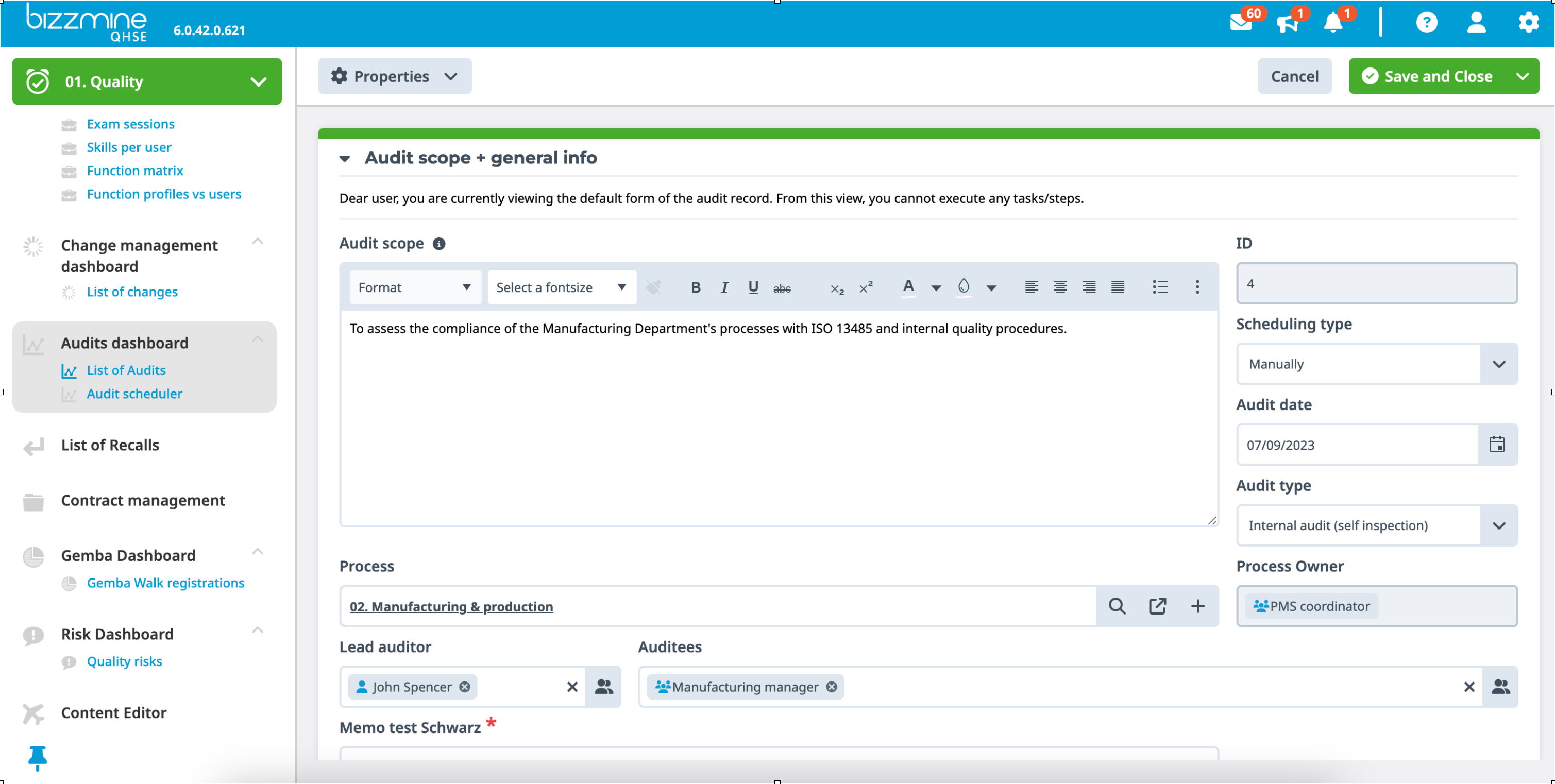
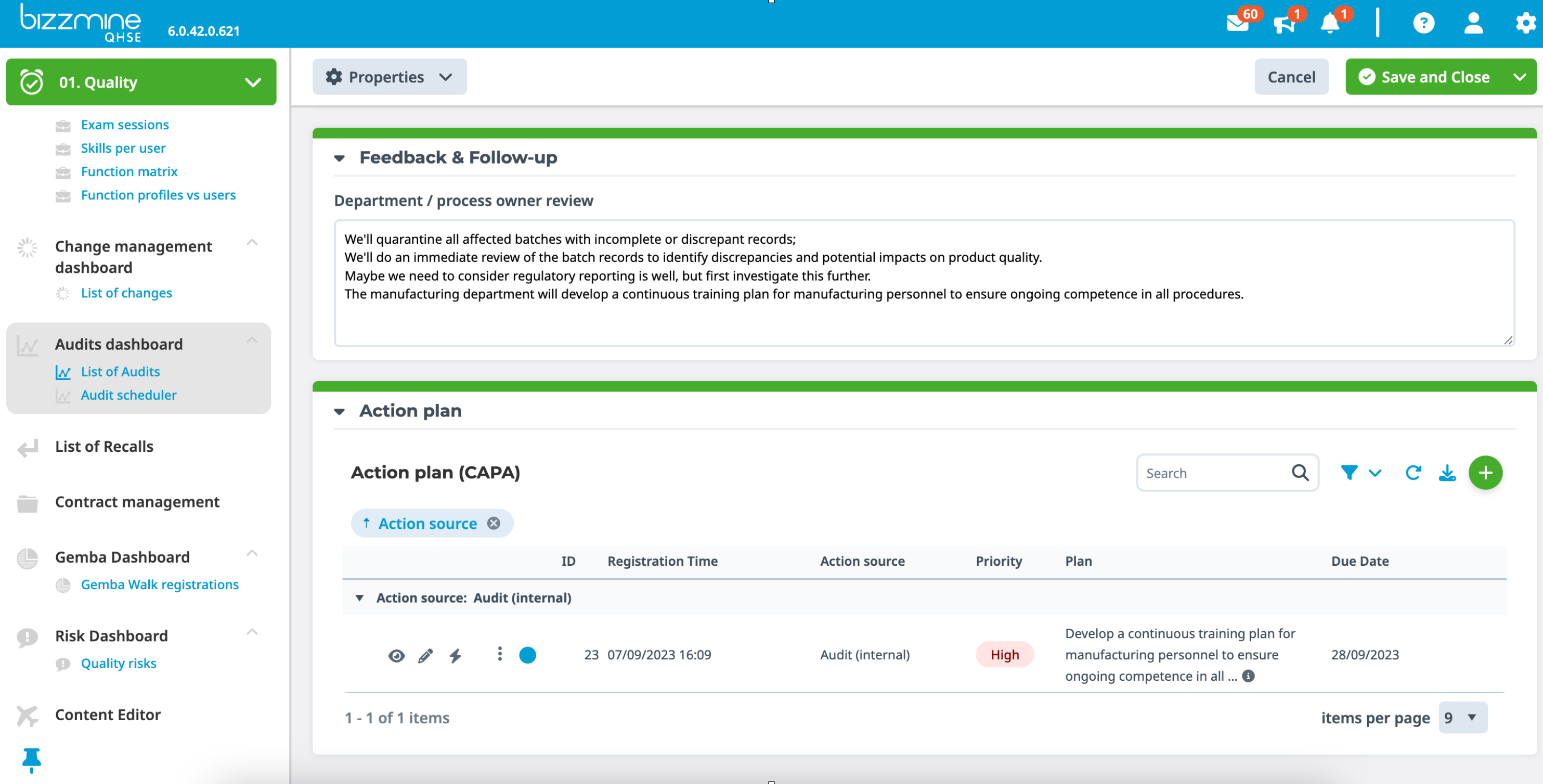
Internal audit software to correct deviations for continuous improvement
Identify, analyse, and track any potential nonconformities discovered during an internal or external audit.
In order to accomplish continuous improvement, initiate corrective actions (CAPA) to fix and prevent potential future deviations, which can be supported with compliance audit software.
Workflow ensures that assigned users receive automatic reminder notifications of tasks, which can be communicated via e-mail.
Prepare yourself for your next audit
Easy access and enhanced communication with audit management tools
All users have simple access to the audit process and may follow up on audits from their laptop, tablet, or mobile phone.
Digital audit management enables auditors and auditees to collaborate seamlessly, allowing them to easily communicate findings and track progress.
Bizzmine is accessible as a cloud service or on your own server, therefore ideal as your internal audit sofware.
"Bizzmine is user-friendly, streamlines daily operations,
and consistently receives praise from auditors
for its compliance and effectiveness."
- Curida Diatec
Choose your industry to learn more
Medical devices
Pharma
Laboratories
GDP Logistics
Food & Beverages
Unlock your potential with Bizzmine
Document Control
You can easily retrieve relevant documents, track changes, and ensure that documentation complies with industry standards.
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)


/Ebook%20cover_Digital%20QMS_EN.png)
























