Calibration Management Software
Bizzmine is the ideal Calibration Management Software tool for planning, organising, and analysing all of your calibration processes.
Full compliance with a Calibration Management System
Calibration Management Software makes your job easier, faster and more regulatory-compliant.
It aids in the quantification and reduction of measurement system variations.

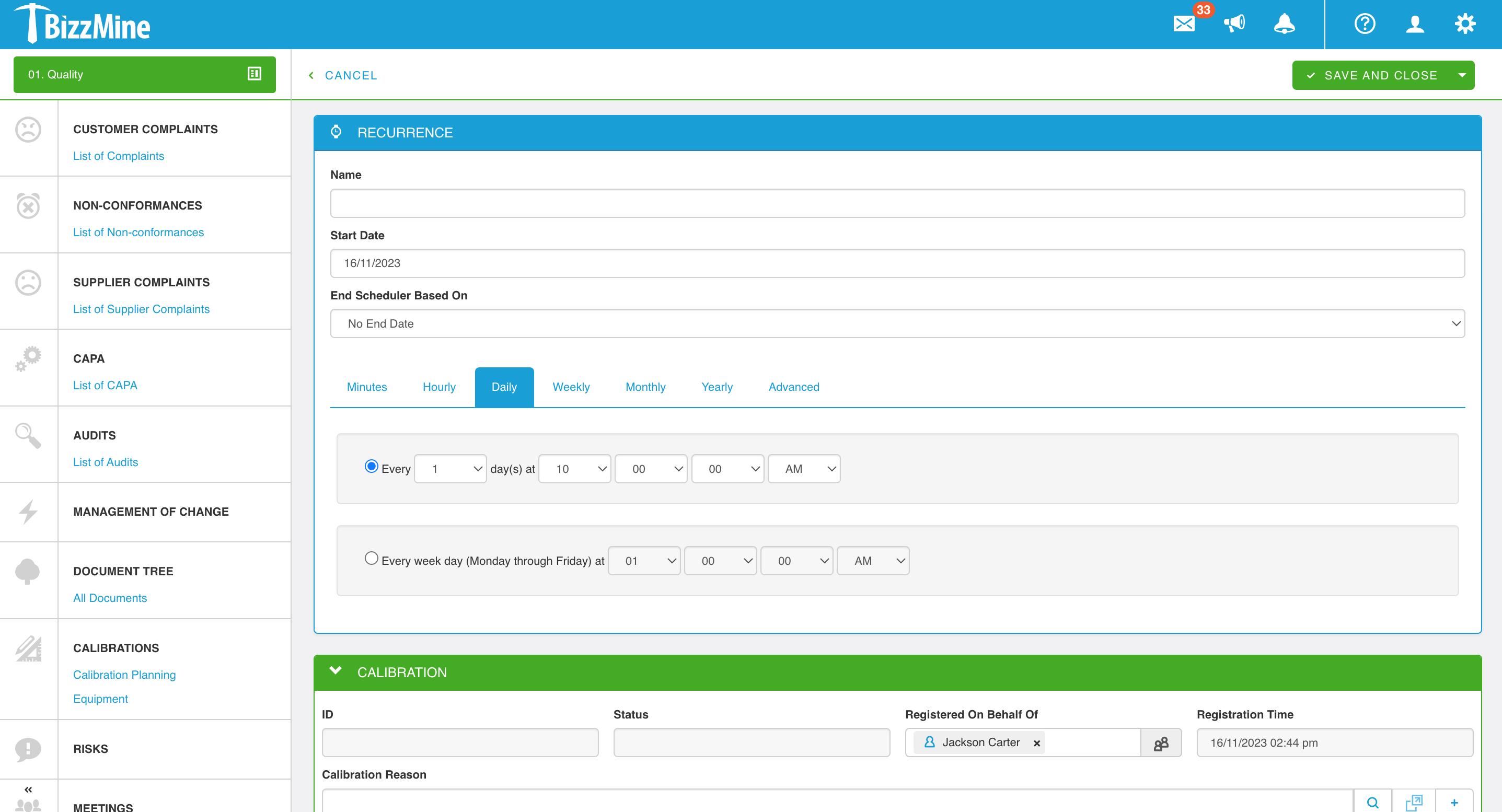
Calibration Management Software to create and schedule
Register all relevant data in the customisable calibration form, and attach (scanned) reports or images to document the findings.
Create, schedule, and allocate calibration engineers work orders. Follow-up can be performed via a computer, smartphone, or tablet. Easily, with the right Calibration Management System.
Plan, organise and analyse Calibration management easily
Correct and improve with Calibration Management Software
Determine, analyse, and track all potential deviations.Launch corrective actions (CAPA) to remedy and prevent future errors in your calibration software.
Deadlines are used to keep track of the intuitive workflow process. When tasks become late, designated users receive automatic reminder notifications.
"Our calibration systems such as scales and sterilisation machines must be validated at specific intervals to ensure they meet standards and that deviations are allowed. This is all fully documented in Bizzmine."
- Staxs
Choose your industry to learn more
Medical devices
Pharma
Laboratories
GDP Logistics
Food & Beverages
Unlock your potential with Bizzmine
Document Control
Ensure that calibration certificates, procedures, and records are properly stored, version-controlled, and easily accessible for audit and review.
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)


/Ebook%20cover_Digital%20QMS_EN.png)





































