GMP compliant software for life science companies
Discover how pharmaceutical manufacturing companies become and stay GMP compliant with a quality management system.
Join 1000+ companies who trust Bizzmine
How our QMS for pharmaceutical companies guarantees
Being fully GMP compliant without any paper based hassle

Paper is in the grave
Our digital QMS offers real-time access, automated updates, and reliable compliance tracking. Tailored to your specific needs for seamless quality management.
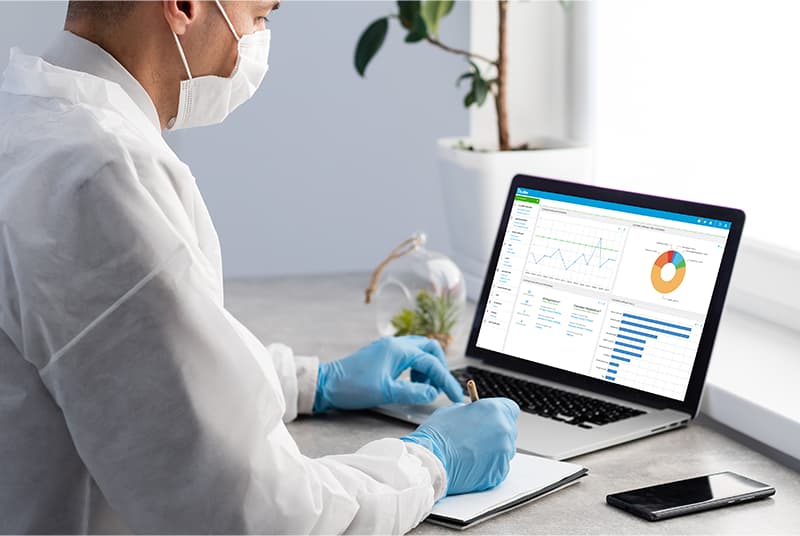
Easily auditable quality processes
Avoid non-compliance risks like product removal and bans. Achieve FDA and EMA GMP certification with ease.

Assurance of quality
Build a better risk management system designed to identify, assess, monitor, and treat risks effectively. Ensuring consistent compliance and superior product quality.
"Bizzmine brings transparency, compliance and traceability of our quality registrations."
- Customers like Carbogen AMCIS rely on Bizzmine's eQMS for ensuring GMP compliance.

15 Requirements you need to find in a solid eQMS for GMP
A proper system is your way to compliance in quality management. Explore how a software solution can revolutionise your digital QMS in any business environment.
Our GMP compliant software features
GAMP5 validated GMP software for quality management according to international standards.
Meet all quality requirements according to cGMP and FDA in one flexible platform.
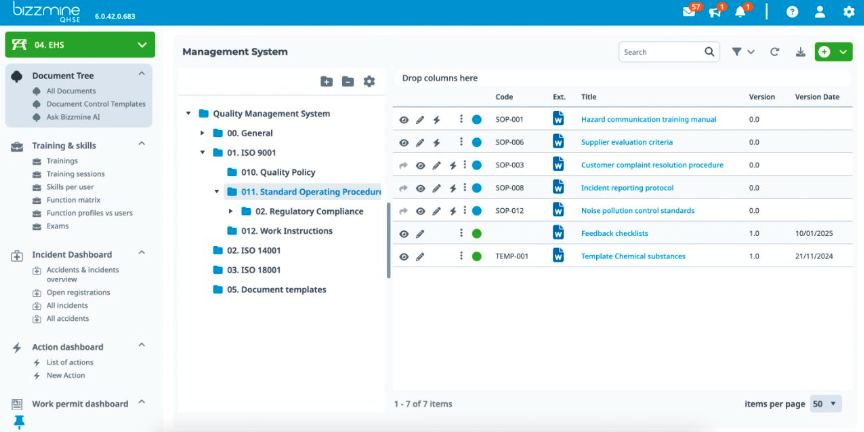
Digitise and streamline multiple interconnected quality processes
Including: Corrective and Preventive Action (CAPA), non-conformances, audits, risk management, document control, Management of Change (MoC), calibration management, and training management.

Seamlessly prepare for GMP certification and compliance audit
• Automate compliance processes to reduce manual work and human errors
• Manage documentation efficiently for easy access and audit readiness. Streamline your workflows
• Streamline workflows to ensure consistent GMP certification
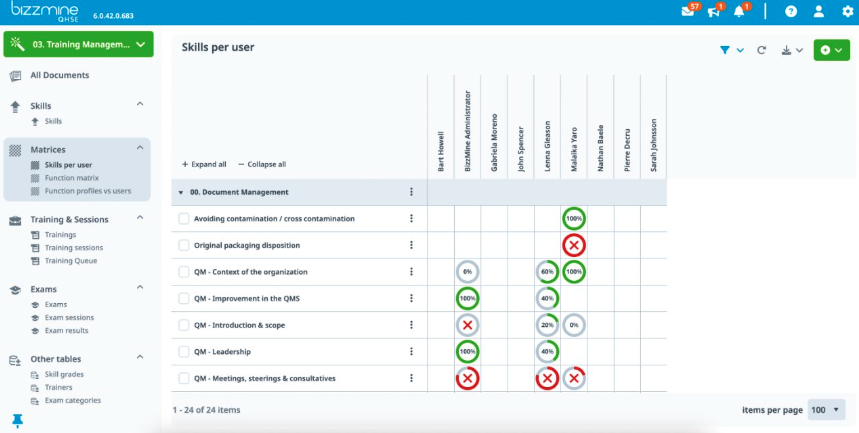

- - Compliance and excellence
- Achieve and maintain compliance and excellence in manufacturing processes. Ensure pharmaceutical drugs are consistently produced and controlled.
- - Tracking en building KPI's with follow-up
- Grant access persomission to the right person at the right level. Have your audit revisions ready in a few business days.

.jpg?width=160&height=103&name=Gettapp%20Review%20(1).jpg)
1000+
Customers
More than 1000 companies use our QHSE software to easily manage Quality and Safety processes.
45
Countries
We provide innovative solutions in over 45 countries worldwide, empowering businesses across diverse industries.
80+
Team
Our team of over 90 talented professionals work together to deliver exceptional QHSE software and services.
Hi, how can we help you to become GMP compliant in the life science industry
What to expect in the demo about our QHSE software?
Introduction about the QMS platform and its functions
How our GMP software solution can help your company to keep track on data, historical records and data (actions) to contribute to your quality strategy
Personal answer to your detailed questions about GMP compliance software for your organisation
















.png)





