Healthcare Quality Management Software
Bizzmine dramatically reduces your time to manage and run clinical audits and other compliance activities within your departments, with its healthcare quality management software.
Achieve seamless healthcare operations with our healthcare QMS, ensuring compliance with industry regulations and improving patient safety.

Regulatory compliance
Bizzmine’s QMS for healthcare is designed to help healthcare organisations comply with all necessary regulations, such as ISO standards in Pharmaceutical (GMP / MHRA Compliance), Pathology (ISO 15189, ISO 22870) and other ISO’s, ensuring patient safety and efficient medical practices. The system streamlines documentation, reduces administrative burdens, and ensures adherence to healthcare standards.

Operational efficiency
Healthcare organisations can streamline and digitise their workflows with Bizzmine’s flexible QMS. Manage corrective and preventive actions (CAPA), audits, incidents, and non-conformities seamlessly, reducing manual efforts and minimising errors. This enables a more responsive and agile healthcare environment focused on delivering the best possible care.

Enhance patient safety
Our QMS supports healthcare providers in managing risks effectively. By tracking incidents, improving documentation, and identifying potential hazards early, Bizzmine ensures that patient safety remains the top priority. With real-time visibility and structured workflows, healthcare institutions can act proactively to safeguard both their patients and reputation.
"Creating and validating automated business processes have probably never been easier.
- Our customers rely on Bizzmine's eQMS for ensuring compliance in healthcare.
Our Healthcare Quality Management Software
With Bizzmine, you have a flexible QMS platform designed for healthcare that meets international standards such as ISO 13485 and FDA requirements. You can manage all quality and compliance needs in one system, ensuring the highest levels of patient safety and operational efficiency.
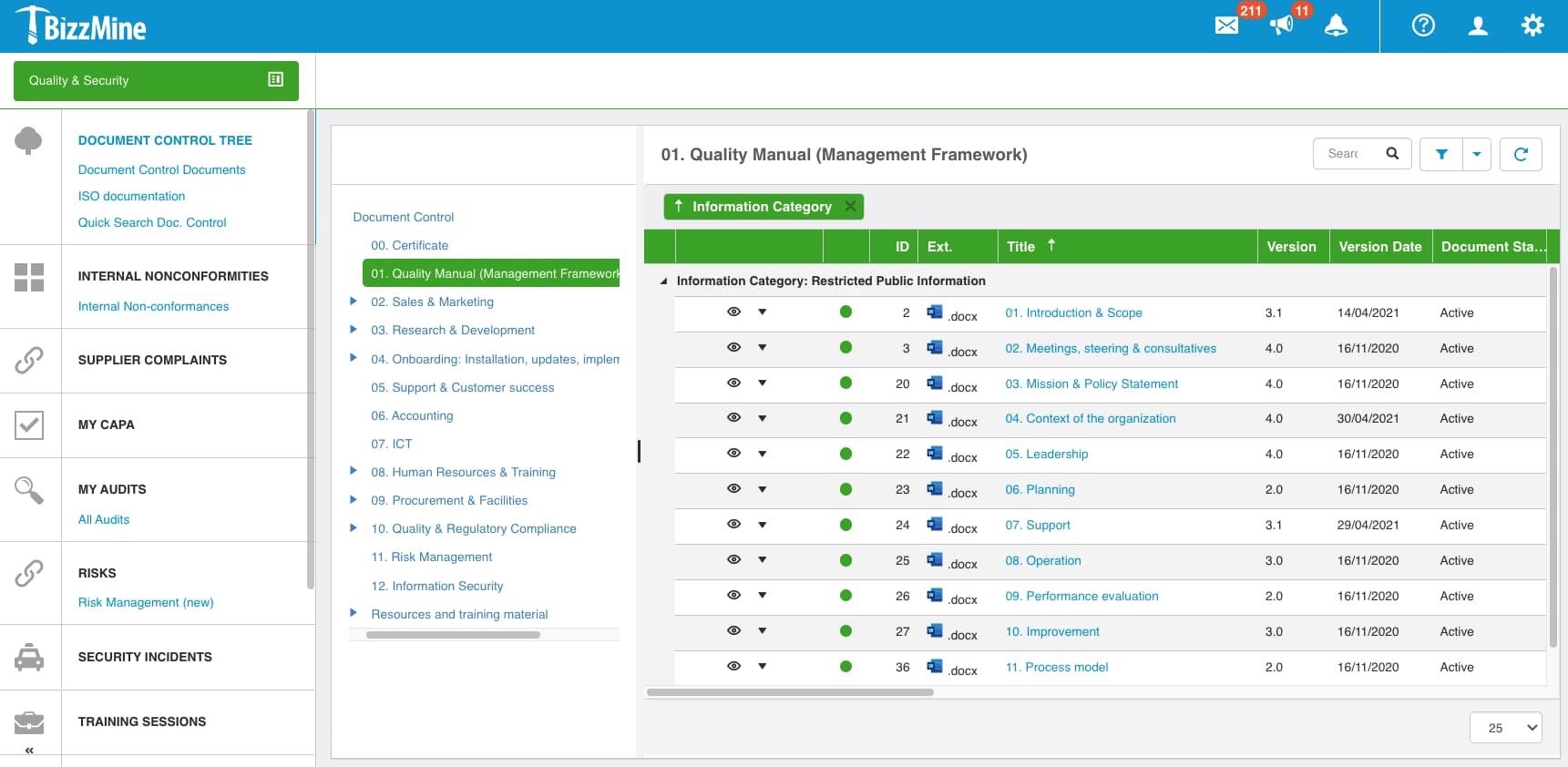
Digitise and streamline multiple interconnected quality processes, including Corrective and Preventive Action (CAPA), non-conformances, audits, risk management, document control, incident management, and training management.
Enhance Healthcare Compliance and Efficiency with healthcare QMS
An eQMS in healthcare can significantly improve overall quality, safety, and compliance while also enhancing operational efficiency and cost-effectiveness.
The software will manage clinical governance in departments such as: Pharmaceutical (GMP / MHRA Compliance), Pathology (ISO 15189, ISO 22870), Radiology (QSI), including other departments such as Blood and other ISO’s.
Implementing a Quality Management System helps you to organize and link numerous processes:
- Clinical Audits and Audit Schedules
- Document Control, Document Changes, Training and Exams
- Non-Conformances, Positive Events and Deviations
- CAPA (Corrective Actions & Preventative Actions)
- Change Control and Equipment Management
- Risk Management
- Supplier Management

eQMS for Healthcare
Ensure compliance with regulations like Pharmaceutical (GMP / MHRA Compliance), Pathology (ISO 15189, ISO 22870), Radiology (QSI), including other departments such as Blood and other ISO’s.
Pre-validated software solution, tailor made for the healthcare industry.
How can we help with your healthcare compliance?
What to expect:
Introduction about the platform and its healthcare QMS features
Advice and onboarding with our most-fitting template/demo for your healthcare environment
Personal answers to your detailed questions about ISO regulations within healthcare
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)



/Customer_Apotheek%20A15_Logo.png)
/Customer_AZ%20Sint%20Jan_Logo.png)
/Customer_CHU%20Li%C3%A8ge_Logo.png)
/Customer_Dobber%20Healthcare_Logo.jpg)
/Customer_Emma%C3%BCs_Logo.jpg)
/Customer_Health%20in%20Code_Logo.jpg)
/Customer_NHS%20GIG%20Cymru_Logo.webp)
/Customer_NHS%20Warrington%20and%20Halton_Logo%20.webp)

/Ebook%20cover_Digital%20QMS_EN.png)
