GMP Compliant Software
How do pharmaceutical companies become and stay GMP compliant with a quality management system?
Discover our GMP compliant software features for Quality manufacturing in the Pharmaceutical Industry.
Achieve seamless pharmaceutical manufacturing through GMP compliant software for your Quality management system

Speed-to-market
Use our GMP compliant software to be ensured at every step of the process. Manage risks and quality efficiently, allowing you to drastically reduce the time to market. Ensure high customer satisfaction and seamless regulatory adherence in pharmaceutical manufacturing.

Stay GMP compliant
Stay GMP compliant and audit ready with easily auditable quality processes. Avoid non-compliance risks like product removal and bans. Achieve FDA and EMA GMP certification easily. Our solution is also 21 CFR Part 11 & Annex 11 compliant, enhancing data security and traceability.

Assurance of quality
Build a better risk management system designed to identify, assess, monitor, and treat risks effectively. Tailored to your specific needs for seamless quality management, our GMP compliant software enhances your Pharmaceutical Quality System (PQS), ensuring consistent compliance and superior product quality.
"Bizzmine brings transparency, compliance and traceability of our quality registrations."
- Our customers rely on Bizzmine's eQMS for ensuring GMP compliance.
Our GMP compliant software features
With Bizzmine you have GAMP5 validated GMP software for quality management according to international standards. You can meet all quality requirements according to cGMP and FDA in one flexible platform.
Digitise and streamline multiple interconnected quality processes, including Corrective and Preventive Action (CAPA), non-conformances, audits, risk management, document control, Management of Change (MoC), calibration management, and training management.
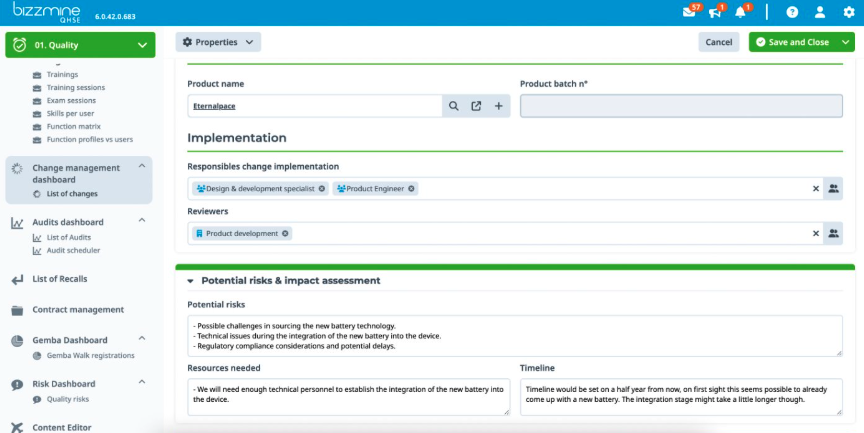
Compliant at all times in a pharmaceutical environment with our GMP software
Ensure your manufacturing processes meet the highest standards of quality and regulatory compliance. Our eQMS software integrates industry best practices, ensuring continuous compliance with cGMP. Bizzmine supports thorough documentation practices, enhancing traceability and accountability.
With a robust quality management system for GMP compliance in one flexible platform your products not only meet but exceed all quality requirements and regulations according to cGMP and FDA.

Seamlessly prepare for GMP certification and compliance audit
Ensure your readiness for GMP certification and audits with our GMP compliant software, specifically tailored for the pharmaceutical industry.
Our GMP software facilitates continuous compliance by automating processes, managing documentation, and streamlining workflows to ensure your operations consistently meet GMP certification requirements. Stay ahead with our comprehensive solution that ensures your facility remains GMP certified at all times.

GMP compliant software for regulated life sciences in manufacturing
Ensure compliance and excellence in every step of your manufacturing process with our comprehensive GMP compliant software solution, tailored for the pharmaceutical industry. With our solution, pharmaceutical drugs are consistently produced and controlled to meet the highest standards of GMP compliance throughout the production cycle.
Pre-validated software solution, tailor made for the pharmaceutical manufacturing industry.
How can we help with your GMP compliance?
What to expect:
Introduction about the platform and its functions
Analysis of your URS
Advice and onboarding with our most-fitting template/demo for your business context
Personal answers to your detailed questions about GMP compliance software for your organisation
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)

















/Ebook%20cover_Digital%20QMS_EN.png)
