Change Control Software
Reduce the risks of change, improve its implementation, and ensure compliance by using software for Change Control.
With Change Control software, Management of Change (MoC) is quicker, easier, and more reliable.
Automate and control with Change Management Software
Take quality decisions regarding changes in a proactive way. Make adjustments with the least amount of risk and impact possible. Determine, monitor, and document all change risks and their impact on your organisation.
Control the change process effectively regardless of where the issue causing the change arises or how many departments the proposed change affects.
Reduce redundancy and manual labour. Change approvals and notifications regarding any event linked to a change can be automated and managed. Task notifications are automatically issued to the stakeholders who oversee each task. Using industry best practices, tracked and approved modifications may be released and implemented quickly. Easily, with regulatory Change Management Software.
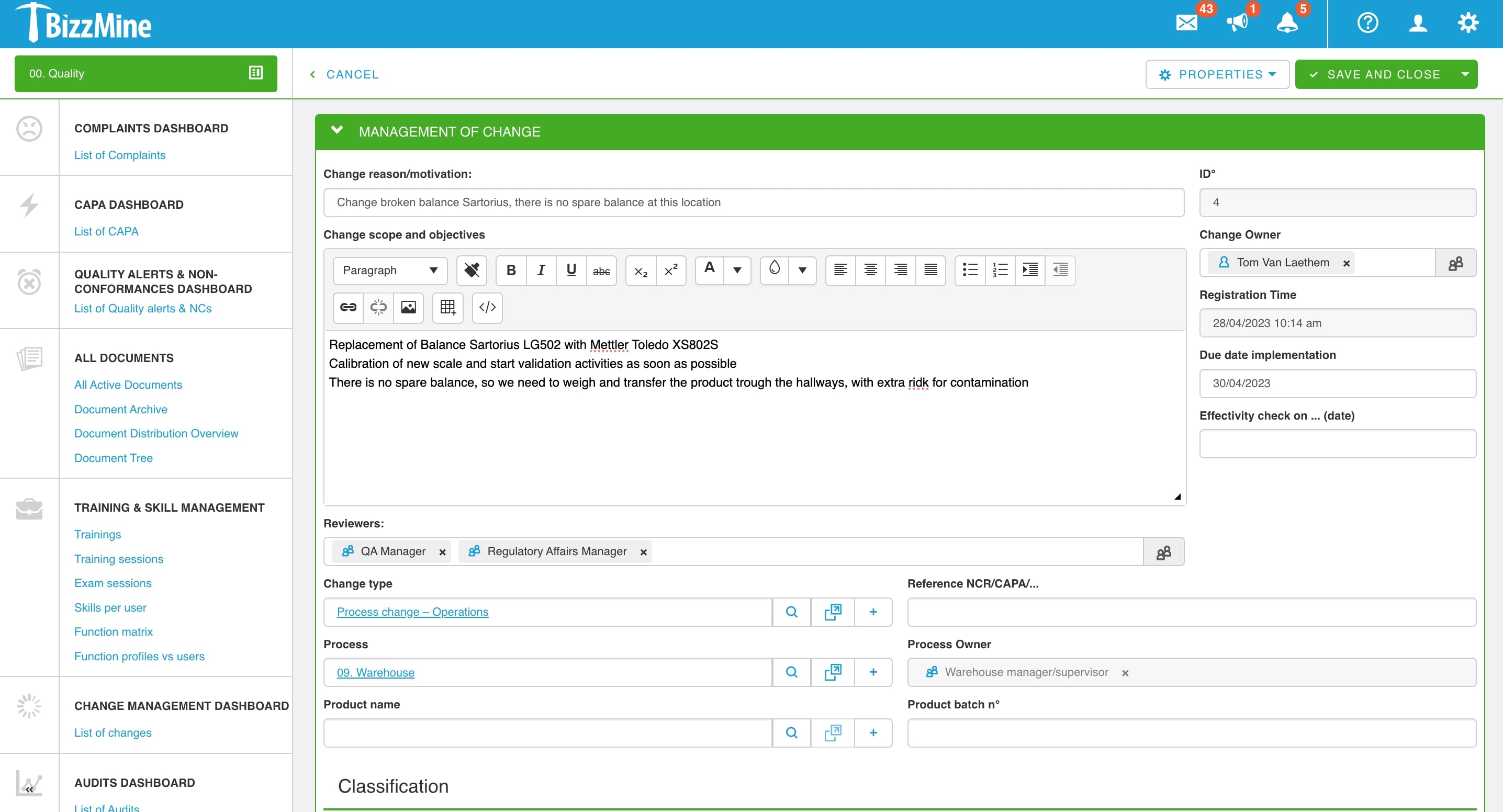
Change Management Software for continuous improvement
Any quality item, such as change records, documents, hazards, CAPAs, customer or supplier complaints, audits, and non-conformances, can be linked.
Integrate your training management in your Change Management Software. Any modification to a document or process that requires new training will instantly trigger training, skills, and exams. You can train colleagues on changes as they occur in real time by connecting your complete quality system.
The interaction with the document control module simplifies the integration of required documents and provides full traceability throughout both modules.
Reduce the risks of change
Change Management Software to be compliant and audit ready
Because you can see what was changed, why and when it was changed, and what was impacted by a change, the Change Control process offers complete traceability.
Your quality system is constantly available for inspections and audits, allowing your company to easily meet auditing and compliance needs.
With time-stamped audit trails and electronic signatures, you can stay in compliance with regulatory requirements and standards like ISO and GxP while also meeting FDA's 21 CFR Part 11 and Annex 11 requirements. Easy, with the right regulatory Change Management Software.
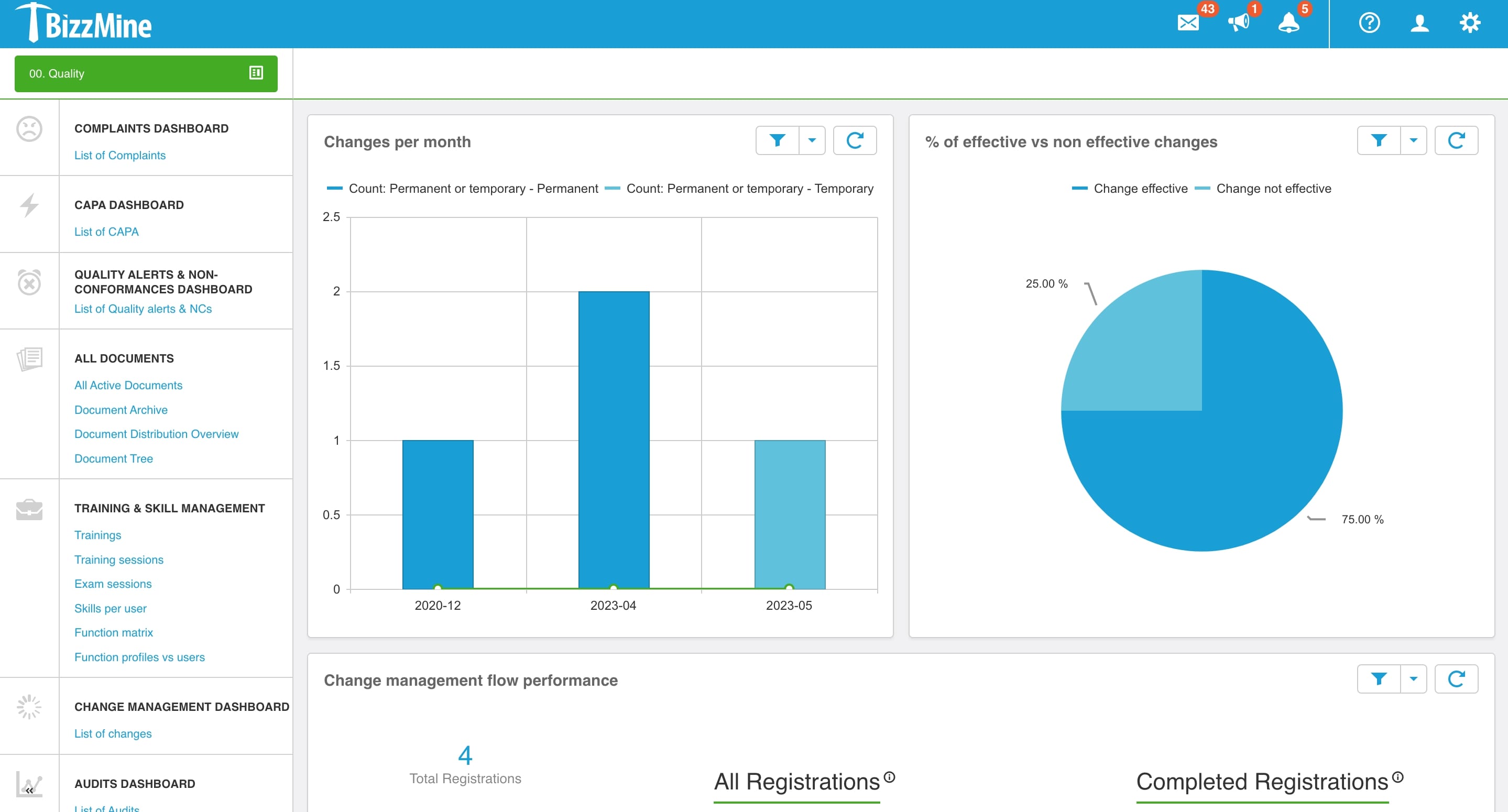
Continuously monitor the effectiveness of the change with Change Management Software
Set up automated procedures and notifications to let IT and business stakeholders understand and communicate more clearly. Handle changes independently with unique and customisable workflows, allowing you to operate the way you want. With automatic notifications in your Change Control Software, you can improve communication.
"With a good preparation on the design of the forms and workflows, Bizzmine was able to implement both CAPA and MoC application within two days."
- Carbogen AMCIS
Choose your industry to learn more
Medical devices
Pharma
Laboratories
GDP Logistics
Food & Beverages
Unlock your potential with Bizzmine
Document Control
Updated documents are created, approved, and distributed in a controlled manner, ensuring that employees have access to the latest versions.
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)


/Ebook%20cover_Digital%20QMS_EN.png)





































