Digital QMS for Medical Devices
Implement a Medical device QMS in your organisation to improve efficiency, compliance with ISO13485 and overall product quality.
Achieve seamless processes with our Medical Device QMS, ensuring compliance with ISO 13485 and FDA regulations.

ISO 13485 compliance
Bizzmine is designed specifically to help medical device companies meet stringent regulatory requirements, including ISO 13485 and FDA standards. Our platform ensures that your quality management processes are fully compliant, reducing the risk of non-conformance and regulatory penalties, while maintaining the highest level of product safety and quality.

Flexible and scalable
Whether you are a small startup or an established medical device manufacturer, Bizzmine's Medical Device QMS adapts to your business. Its customisable workflows allow you to streamline critical processes like CAPA management, audits, and document control, ensuring your operations run efficiently as you scale.

Process automation
Bizzmine automates time-consuming manual tasks, enabling you to focus on innovation and production. By digitising key quality processes, such as risk management and training, our platform increases operational efficiency while maintaining compliance, helping you stay ahead in the competitive medical device market.
"With Bizzmine, we feel that we have a secure and homogeneous environment to maintain, order, and standardise our ISO documents.
The software really helped us get through our process of becoming ISO certified in a short time and with less work."
- Tiki Safety
Our QMS software Medical Devices
As a Medical Devices company, you help improve health and quality of life by innovating and accelerating high-quality research.
Implementing a Quality Management System, like ISO13485, helps you to organise and link numerous processes.
In Bizzmine you can easily digitise and automate these quality processes: Document Control (SOP), Management of Technical Files, Internal and External Audit Management, Risk Management, Corrective and Preventive Action (CAPA), Non-conformance Management, Post-Market Surveillance, Complaint Management, Incident Management (Vigilance), Calibration Management, Management of Change (MoC), Training Management, Design Control.
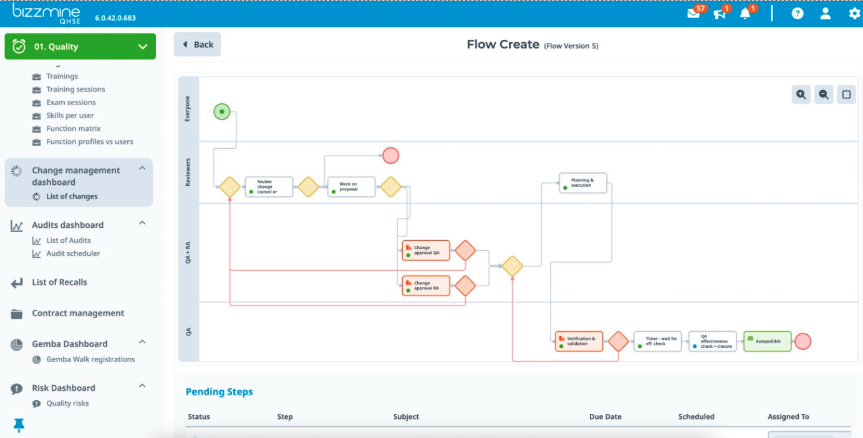
Software according to ISO 13485 and FDA
With Bizzmine, you have a flexible QMS designed specifically for the medical device industry, ensuring compliance with ISO 13485 and FDA regulations.
This level of compliance helps you enhance product safety, streamline operations, and improve market access. By leveraging Bizzmine’s prebuilt workflows for document control, risk management, and CAPA, you can simplify your Quality Management System validation process.
As your business grows, Bizzmine’s digital QMS scales with you, efficiently managing increasing documentation, regulatory requirements, and quality processes to ensure long-term success and compliance.

QMS Software Medical Devices aligned with regulatory standards
With Bizzmine, you can streamline your Medical device QMS using our prebuilt, validated eQMS processes, ensuring compliance and efficiency. Our digital QMS scales effortlessly as your business expands, improving performance and opening new market opportunities
Pre-validated software solution, tailor made for companies in Medical Devices.
How can we help you?
What to expect:
Introduction about the platform and its functions
Advice and onboarding with our most-fitting template/demo for your business context
Personal answers to your detailed questions about our software for you as an organisation in Medical Devices
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)




















/Ebook%20cover_Digital%20QMS_EN.png)

