Complaint Management System
With Bizzmine you can easily track, investigate, and correct your customer and supplier complaints, which helps in your complaint handling.
Efficient follow-up with the right Complaint Management System
It is simple to handle complaints with Bizzmine as your complaint management software. You can track, investigate, and correct customer complaints, supplier complaints, and internal deviations more effectively. Compliance with ISO standards such as ISO 9001, ISO 13485, ISO 17025, and FDA requirements is guaranteed.
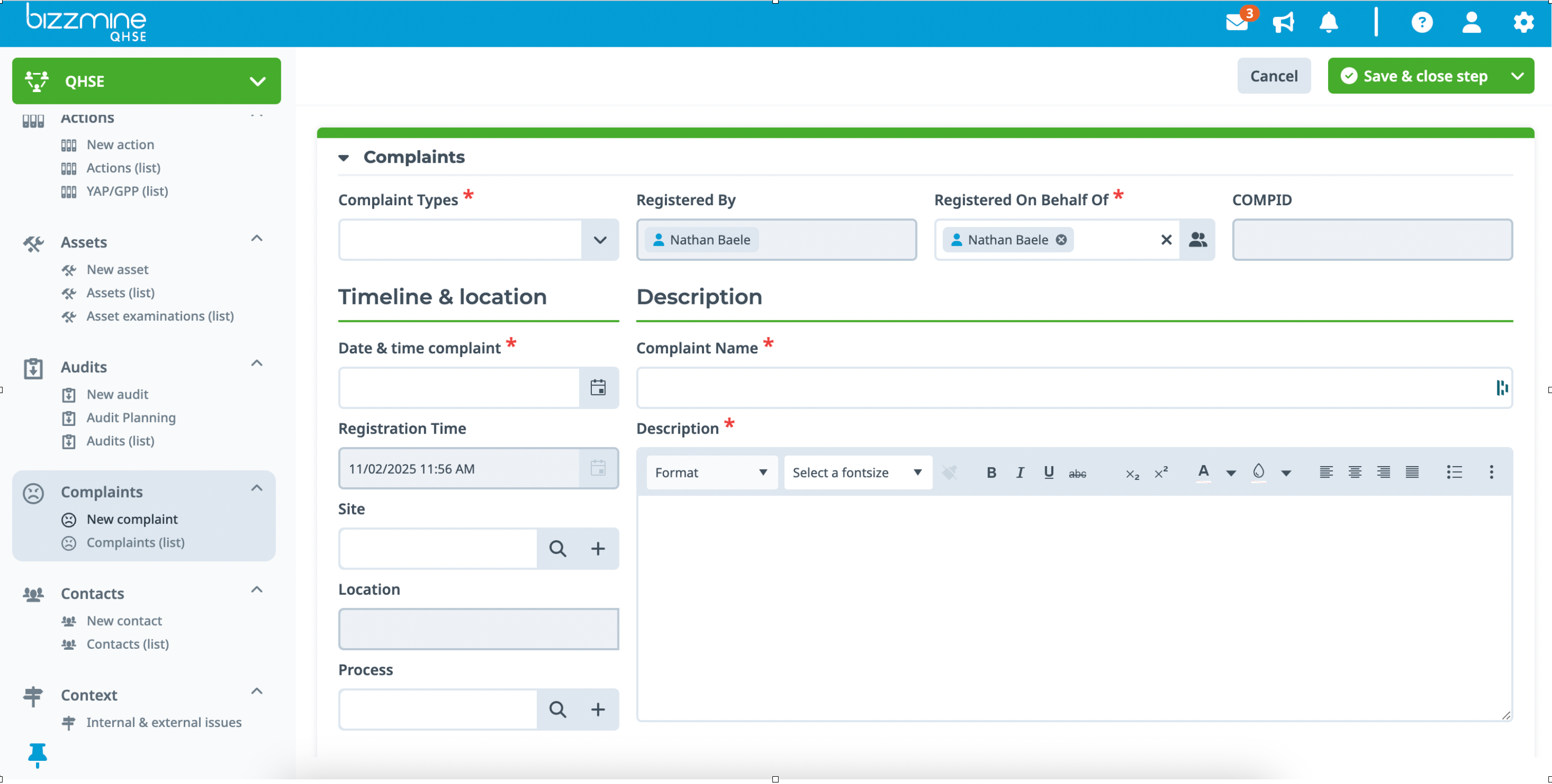
Easy registration in your Complaint Management System
Allow your team to consistently register complaints or non-conformances from their mobile phones or tablets. Connect a contact form so that clients can submit problems directly from your website. Alternatively, your CRM or ERP application can initiate the complaint handling procedure.
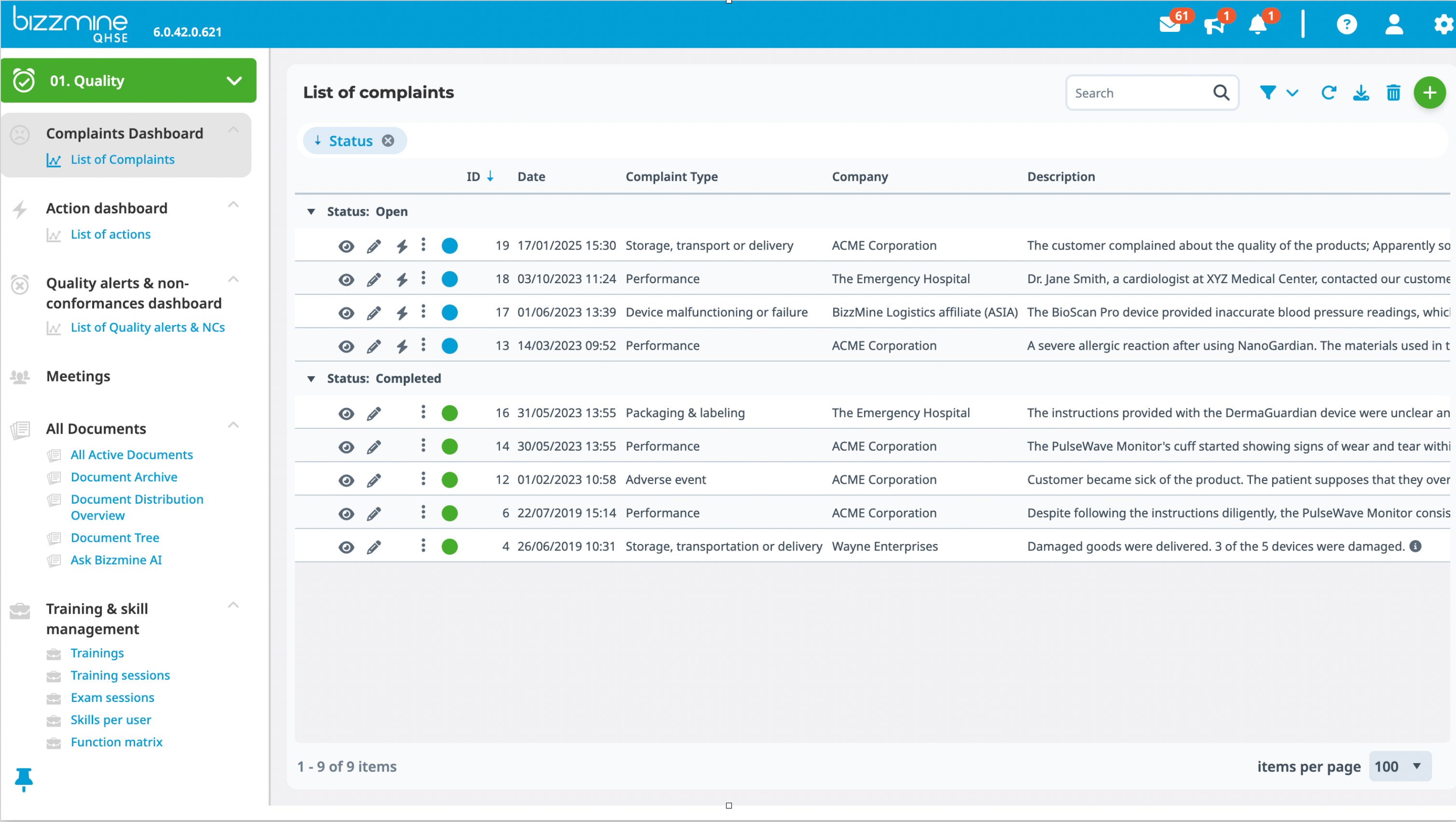
You can see the exact status of each complaint, as well as who did what and when. That is complaint handling at its finest.
Explore Bizzmine’s QHSE Platform
Correct and improve in your Complaint Management System
Launch corrective actions (CAPA) to avoid further complaints and make complaint handling effective.
Register all relevant data in the customizable complaint form, and attach reports or images.
Take benefit of the chance to increase consumer loyalty.
To increase product quality and client retention, use strong Pareto diagrams and Trend analytics.
You can also make the most of your management reviews with these insights.
"Bizzmine brings transparency, compliance and traceability of our quality registrations."
- CARBOGEN Amcis
Choose your industry to learn more
GDP Logistics
Pharma
Oil and Gas
Transform safety from a burden to an opportunity. Centralise processes, reduce manual work, and ensure compliance.
Medical Devices
ISO 13485 compliance is ensured by improved document control, CAPA, audit, and training management.
Food & Beverages
Chemicals
Our digital QHSE management centralises processes and ensures compliance with chemical standards.
Unlock your potential with Bizzmine
Training Management
Ensure that employees are adequately trained to prevent the recurrence of complaints due to human error or misunderstanding of procedures.
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)


/Ebook%20cover_Digital%20QMS_EN.png)























